The silver melt temperature is 961°C (1763°F), which is lower than metals like gold and copper but higher than aluminum. Here’s how the silver melt temperature compares to some common metals:
Copper: 1084°C
Aluminum: 660°C
Iron: 1482–1593°C
Understanding the silver melt temperature, along with the melting points of other metals, is crucial. It helps in selecting the right materials for tasks such as jewelry making, metalworking, or industrial applications. This knowledge ensures precision and prevents damage during heating processes.
Key Takeaways
Silver melts at 961°C (1763°F). It melts easier than gold or platinum.
Knowing melting points helps pick metals for jewelry and tools. It prevents mistakes and damage.
Mixing metals or impurities can change how hot they melt. This is important for good results.
Silver melts about 300°F hotter than when it softens. Heat control matters when crafting.
Picking metals by melting points improves strength and looks in projects like jewelry or machines.
Silver Melt Temperature

What Is the Melting Temperature of Silver?
Silver melts at about 961.8°C (1763.2°F). At this temperature, silver changes from solid to liquid under normal air pressure. This exact melting point is important for many uses, like making jewelry or working with metals. When mixed with other metals to create alloys, silver's melting point can change slightly. For instance, sterling silver, made of 92.5% silver and 7.5% copper, melts a bit differently.
Knowing silver's melting point helps you handle it properly. Whether you're making detailed jewelry or using silver in factories, understanding its melting point ensures accuracy. It also stops overheating, which can harm the metal or change its properties.
Why Is Silver's Melting Temperature Important?
Silver's melting point is key for many uses. In jewelry making, controlling heat is very important. Silver melts about 300°F higher than its annealing temperature, which is around 1200°F (650°C). Annealing softens silver, making it easier to shape. If overheated, silver can get fire scale or even melt by accident. This shows why careful heat control matters.
In crafting and industry, silver's melting traits decide how it’s used. For example, silver brazing fillers, made of 50% silver, 34% copper, and 16% zinc, use silver's melting point to join materials. Dental alloys with 60-70% silver are strong and safe for fillings. Below is a table showing common silver alloys and their uses:
Alloy Type | Composition | Application Description |
|---|---|---|
Sterling Silver | 92.5% silver, 7.5% copper | Strong and shiny, perfect for everyday jewelry. |
Coin Silver | 90% silver, 10% copper | Used in jewelry and decorative items. |
Dental Alloys | 60-70% silver, 18-25% tin, etc. | Great for dental fillings due to strength and safety. |
Silver Brazing Fillers | 50% silver, 34% copper, 16% zinc | Used for joining metals, showing silver's versatility. |
By learning about silver's melting point, you can complete projects successfully. Whether designing jewelry or working in factories, this knowledge helps you get the best results without damaging the material.
Melting Points of Metals Compared to Silver
Precious Metals (Gold, Platinum)
Gold and platinum are valuable metals often compared to silver. They are used in jewelry and industries. Gold melts at 1,064°C (1,947°F), which is hotter than silver's 961°C (1,763°F). This makes gold better for heat-heavy crafting. Platinum melts at an even higher temperature of 1,768°C (3,214°F). This makes it perfect for high-heat uses like lab tools and car parts.
The melting point differences come from their atomic structures. Platinum has tightly packed atoms and strong bonds, giving it a high melting point. Gold, though less dense than platinum, still melts at a higher temperature than silver. This is because of its strong atomic bonds. Knowing these differences helps pick the right metal for tasks like making jewelry or industrial work.
Industrial Metals (Copper, Aluminum, Iron)
Metals like copper, aluminum, and iron are key in building and manufacturing. Copper melts at 1,084°C (1,983°F), higher than silver. This makes it great for wires and pipes that need heat resistance. Aluminum melts at 660°C (1,220°F), which is lower than silver. Its low melting point makes it easy to shape, so it’s used in cars and planes.
Iron melts between 1,482–1,593°C (2,700–2,900°F). Its high melting point makes it strong for buildings and machines. The table below shows how these metals compare to silver:
Metal | Melting Point |
|---|---|
Aluminum | 660°C (1,220°F) |
Copper | 1,084°C (1,983°F) |
Silver | 961°C (1,763°F) |
Iron | 1,482–1,593°C |
Knowing these melting points helps you pick the right material. Whether you need lightweight aluminum or strong iron, this knowledge ensures success.
Metals with Higher or Lower Melting Points
Some metals melt at much higher or lower temperatures than silver. High melting point metals, like tungsten, rhenium, and iridium, are used in extreme heat. Tungsten melts at 3,422°C (6,192°F), making it great for light bulbs and furnaces. Rhenium and iridium, melting at 3,180°C (5,756°F) and 2,446°C (4,435°F), are used in jet engines and space tools.
Low melting point metals, like lead and aluminum, are easier to work with. Lead melts at 327°C (621°F) and is used in soldering and radiation shields. The chart below shows how different metals compare:
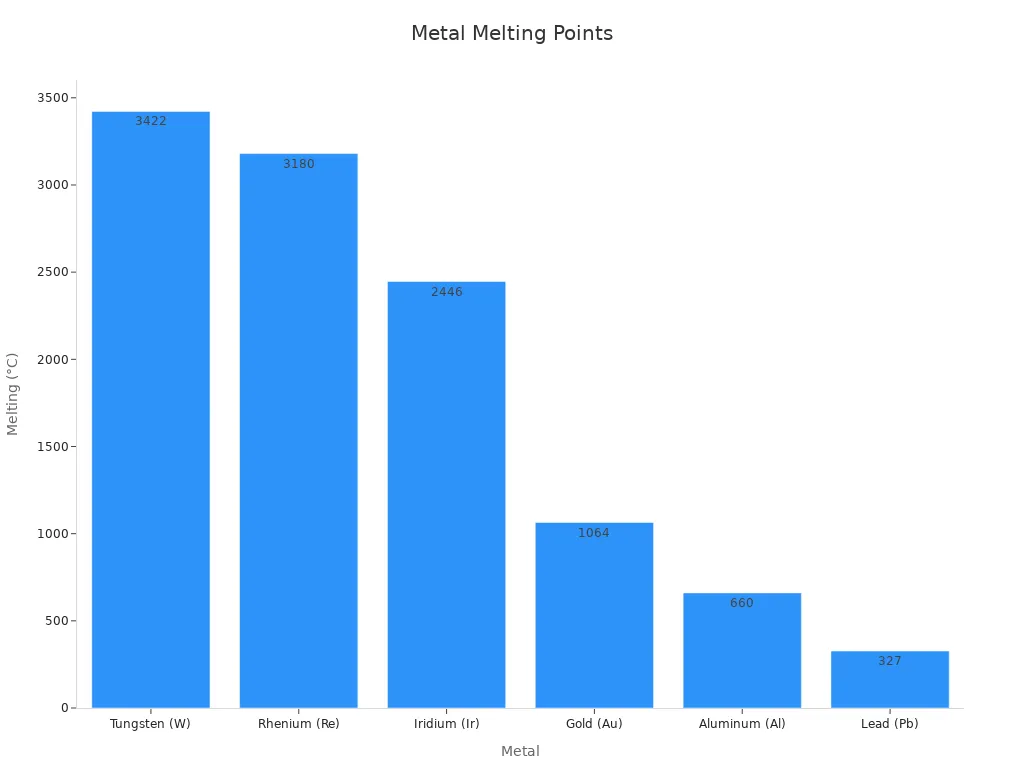
Melting points depend on atomic structure and bonds. Metals with tightly packed atoms and strong bonds melt at higher temperatures. For example, tungsten’s dense structure makes it very heat-resistant. Metals with weaker bonds, like lead, melt at lower temperatures.
Knowing metal melting points helps you choose the right material. High melting points work for extreme heat, while low ones are easier to handle. This knowledge is very useful for many projects.
Factors That Affect Metal Melting Temperatures
How Atomic Structure Impacts Melting
A metal's atomic structure affects how much heat it needs to melt. Metals with tightly packed atoms and strong bonds need more heat, so they have higher melting points. Silver has medium-strong bonds, giving it a melting point of 961.8°C. Gold has stronger bonds because of its half-filled 6s-band, so it melts at 1064°C. Mercury has weak van der Waals forces and a filled 6s-band that doesn’t help bonding. This makes mercury melt at a very low temperature of -38.83°C.
Metal | Melting Point (°C) | Bonding Details |
|---|---|---|
Mercury | -38.83 | Weak van der Waals forces; filled 6s-band doesn’t help bonding. |
Gold | 1064 | Stronger bonds due to half-filled 6s-band. |
Silver | 961.8 | Medium-strong bonds compared to gold and mercury. |
Studies show that atomic changes near neighboring atoms affect metal properties. When temperatures drop, atomic coordination numbers increase. These changes impact melting points and how metals behave, proving atomic structure is key to melting temperatures.
How Impurities and Alloys Change Melting Points
Impurities and alloys can change how metals melt. Pure metals melt at fixed temperatures, but mixing in other elements can raise or lower this. For example, pure silver melts at 961.8°C. Sterling silver, which is 92.5% silver and 7.5% copper, melts at a slightly different temperature because of the copper. Impurities mess up the atomic arrangement, making bonds weaker and easier to break with heat.
Alloys are made to improve things like strength or rust resistance. For instance, silver brazing fillers mix silver, copper, and zinc. They melt at lower temperatures than pure silver, making them great for joining metals in factories. Knowing how impurities and alloys affect melting helps you pick the best material for your work.
Practical Uses of Metal Melting Points
Making Jewelry and Crafts
Knowing metal melting points is important for making jewelry. Silver melts at 961°C (1763°F), making it a top choice. Its lower melting point, compared to gold or platinum, makes it easier to use. Beginners can handle silver better because it needs less heat. This helps avoid overheating and keeps the metal shiny.
Gold melts at 1064°C (1947°F) and is also popular. Its higher melting point makes it great for strong, long-lasting designs. Platinum melts at 1768°C (3214°F) and is used for high-end, tough jewelry. By learning these differences, you can pick the best metal. Choose based on beauty, strength, and how easy it is to work with.
Industrial and Science Uses
In factories, melting points help choose the right metals. Silver’s medium melting point works well for electrical parts and brazing. It conducts electricity well and melts at a manageable temperature. This makes it useful in electronics and tools.
Metals with very high melting points, like tungsten (3422°C), are used in extreme heat. They are found in furnaces, light bulbs, and space equipment. Metals with lower melting points, like aluminum (660°C), are used for lightweight things. These include car and airplane parts.
The need for metals with specific melting points is growing. The table below shows trends in industries and jewelry making:
Metric | Value |
|---|---|
Market Size (2023) | |
Projected Market Size (2032) | USD 850 million |
CAGR (2023-2032) | 3.9% |
Key Industries | Metallurgy, Foundry, Electronics, Chemicals |
Growth Factors | Demand for high-quality metals, advancements in manufacturing technologies |
By understanding these trends, you can see why melting points matter. This knowledge helps you choose the right metal for jewelry or industrial projects.
Silver melts at 961°C (1,763°F). This is hotter than aluminum, which melts at 660°C. It needs more heat, so silver works well for strong items. Gold and platinum melt at 1,064°C and 1,768°C. They handle higher heat, making them great for special uses.
Knowing these melting points helps you pick the right metal. Whether making jewelry or working in factories, this knowledge ensures accuracy. The table below shows how metals compare:
Metal | Melting Point (°C) | Difference from Silver (°C) |
|---|---|---|
1,084 | 122 | |
Gold | 1,064 | 102 |
Aluminum | 660 | -301 |
Lead | 327 | -634 |
Iron | 1,538 | 576 |
By learning about melting points, you can choose materials wisely. This helps you finish projects without errors or damage.
FAQ
What is the melting point of silver in Fahrenheit and Celsius?
Silver melts at 961°C (1763°F). This is when silver changes from solid to liquid under normal air pressure. Knowing this helps you safely use silver in crafts or factories.
How does silver's melting point compare to gold and platinum?
Silver melts at 961°C, which is lower than gold (1064°C) and platinum (1768°C). This makes silver easier to use for jewelry. Gold and platinum need more heat, so they are better for stronger designs.
Can impurities affect silver's melting point?
Yes, impurities or added metals can change silver's melting point. For example, sterling silver (92.5% silver, 7.5% copper) melts at a slightly different temperature. Impurities weaken atomic bonds, making melting easier or harder.
Why is silver's melting point important for jewelry making?
Silver’s melting point tells how much heat is needed to shape it. It melts at 961°C, which is easy for beginners to handle. Controlling heat stops damage like fire scale and keeps silver shiny.
What metals have higher melting points than silver?
Metals like tungsten (3422°C), iron (1482–1593°C), and copper (1084°C) melt at higher temperatures than silver. These metals are used for hot jobs like furnaces, engines, and wires.
Tip: Always check a metal's melting point before starting a project. This helps you pick the right tools and methods for your work.
 LKprototype
LKprototype